
If you have feedback or you find that this document uses some content in which you have rights and interests, please contact us through this link.

Alibaba Cloud accepts no responsibility for any consequences on account of your use of the content without verification.
#Bh3 electron domain geometry professional
We recommend that you consult a professional if you have any doubt in this regard. It is your responsibility to determine the legality, accuracy, authenticity, practicality, and completeness of the content. Such automatically generated content does not reflect the views or opinions of Alibaba Cloud.
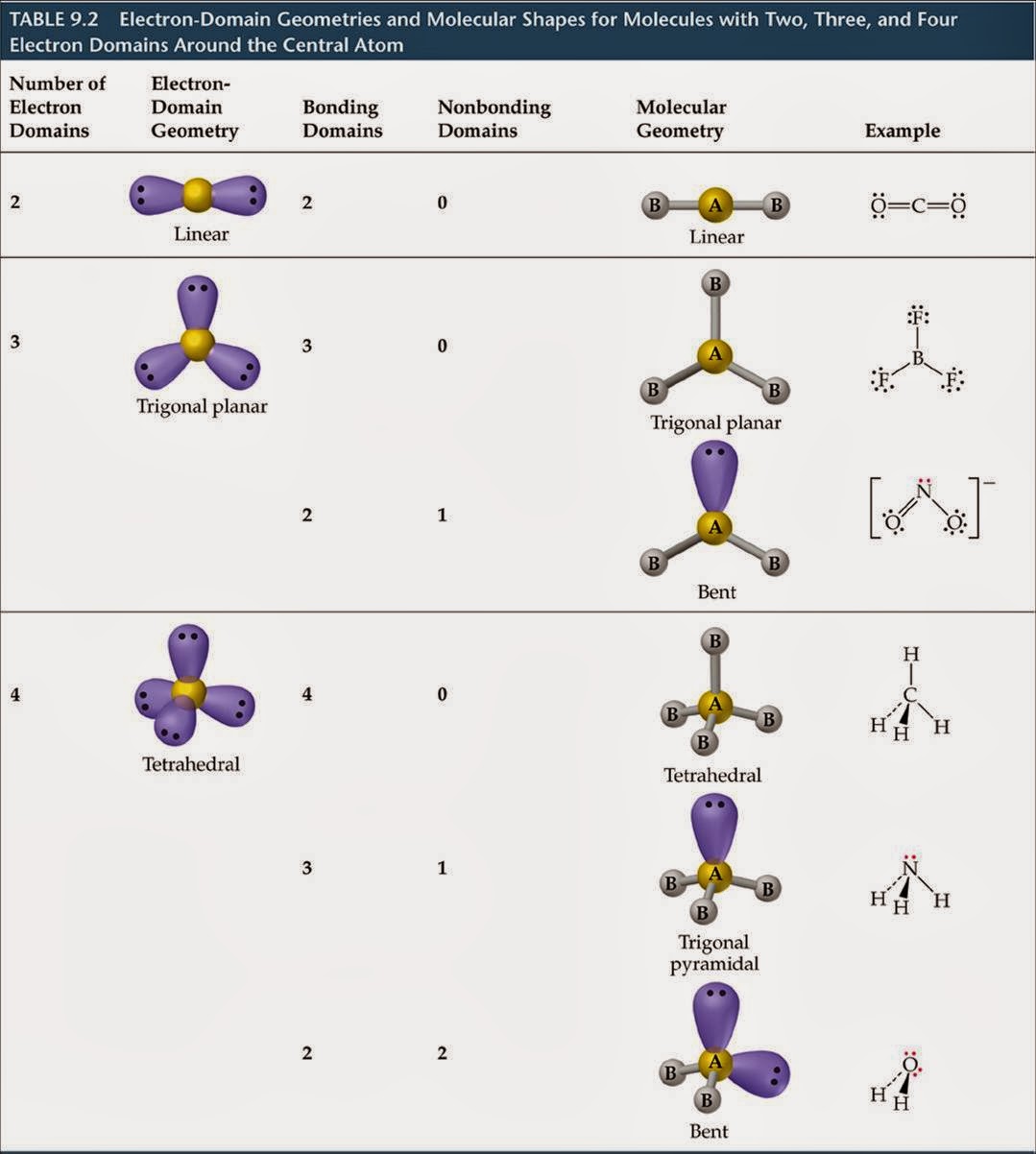
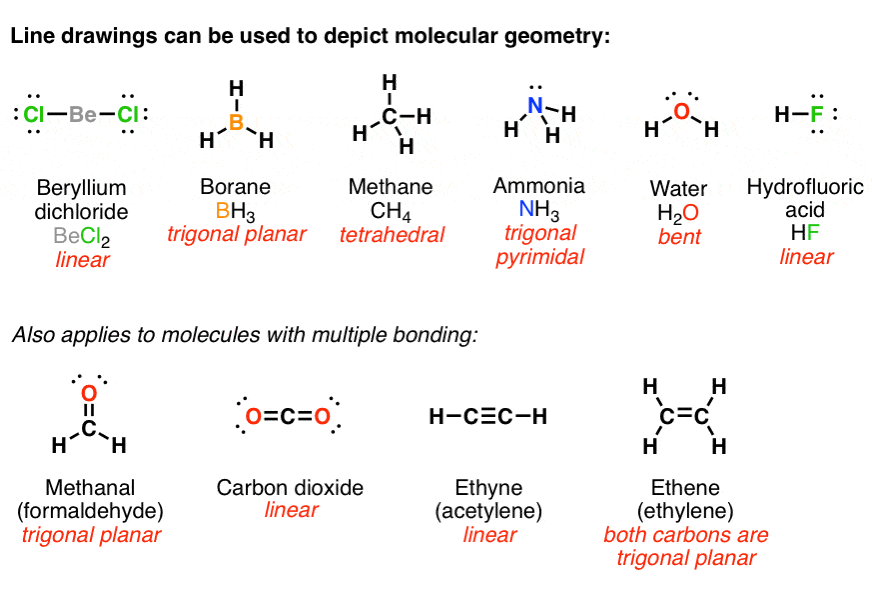
The copyright of the information in this document, such as web pages, images, and data, belongs to their respective author and publisher. This document is automatically generated based on public content on the Internet captured by Machine Learning Platform for AI. You may choose not to use the service if you do not agree to this disclaimer. By using the service, you acknowledge that you have agreed to and accepted the content of this disclaimer in full. Please read this disclaimer carefully before you start to use the service. The sp3 hybridization results in a trigonal pyramidal molecular geometry. This means that the nitrogen atom has four electrons in its outermost shell, and these electrons are arranged in three orbitals. In NH3, the hybridization of the nitrogen atom is sp3. A tetrahedral electron geometry corresponds to 'sp'3 hybridization. Thus, VSEPR theory predicts a tetrahedral electron geometry and a trigonal planar electron geometry. > The Lewis structure of 'CH'3:'-' is The carbanion has three bonding pairs and one lone pair. The hybridization of NH3 is sp3.ĭomains are areas within a molecule that have a particular chemical environment. It is trigonal pyramidal and 'sp'3 hybridized. This means that the NH3 molecule has a permanent dipole moment, with the nitrogen atom being slightly negative and the hydrogen atoms being slightly positive. In the context of the internet, a domain is the unique name that identifies a website. The dipole moment of NH3 is 1.47 Debye.Ī domain is a defined area within which a set of rules or activities apply. This means that the distance between the nitrogen atom and each of the three hydrogen atoms is 1.01 Angstroms. The bond length of NH3 is 1.01 Angstroms. In the context of the Internet, a domain is the unique name that identifies a website. The bond length of NH3 is 1.01 Angstroms.Ī domain is a defined area within which a particular set of rights or privileges exists. This means that the three hydrogen atoms are arranged in a triangle around the nitrogen atom, with the bond angles between them being 107.3 degrees. The bond angle of NH3 is 107.3 degrees.Ī domain is a three-dimensional region of space in which the magnetic moments of the atoms are aligned. The electron domain geometry of NH3 is trigonal pyramidal, meaning that there are three electron domains around the central atom and that the shape of the molecule is pyramid-like. The electron domain geometry of NH3 is trigonal pyramidal.ĭomains are defined as a three-dimensional region in space in which the electron density is significantly higher than the surrounding area.


 0 kommentar(er)
0 kommentar(er)
